|
Chong Kun Dang. (Image: Chong Kun Dang) |
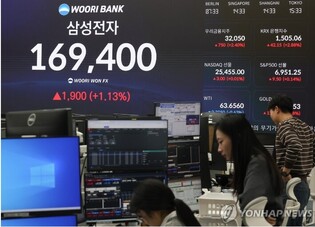
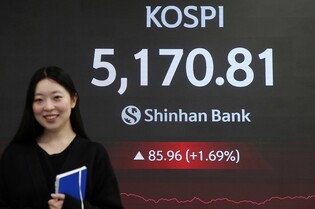
[Alpha Biz= Kim Jisun] Chong Kun Dang announced on May 22 that it has received its first milestone payment following the initiation of clinical trials for CKD-510, a next-generation anticancer drug candidate out-licensed to Novartis.
The milestone, valued at approximately KRW 6.9 billion (USD 5 million), was triggered by Novartis’ submission of an Investigational New Drug (IND) application for CKD-510 to the U.S. Food and Drug Administration (FDA). The specific indication for the trial has not yet been disclosed.
CKD-510 is a selective HDAC6 (histone deacetylase 6) inhibitor. Chong Kun Dang signed a licensing agreement with Novartis in November 2023, which included an upfront payment exceeding KRW 100 billion and a total deal value of up to KRW 1.73 trillion, including development and sales-based milestone payments.
This milestone achievement marks the first visible progress since the out-licensing agreement, helping to alleviate concerns about potential discontinuation or return of the licensed asset.
Chong Kun Dang stated, “We are pleased to see the advancement of CKD-510 into clinical trials and look forward to continued progress through our collaboration with Novartis.”
Alphabiz Reporter Kim Jisun(stockmk2020@alphabiz.co.kr)