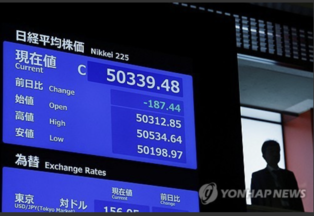
|
Photo = Hugel |
[Alpha Biz= Kim Jisun] Seoul, July 1 — Hugel’s botulinum toxin product Letibot (marketed domestically as Botulax) is making rapid inroads in China, bolstered by its quality credentials and first-mover advantage as the only Korean botulinum toxin officially approved by Chinese regulators.
According to industry sources, Letibot generated approximately KRW 26 billion (USD 19 million) in revenue from China last year, accounting for about 35% of Hugel’s total KRW 75.4 billion sales in the Asia-Pacific region. Financial analysts, including Samsung Securities, forecast that Letibot’s China revenue could surpass KRW 33 billion (USD 24 million) in 2024 if current momentum continues.
This performance comes four years after Hugel secured approval from China’s National Medical Products Administration (NMPA) in October 2020, making it the first and only Korean product in the highly competitive Chinese botulinum toxin market.
Industry data estimates Letibot’s current market share in China at approximately 14%, placing it in third place among approved products. Hugel aims to raise its share to 20–25% by 2027, tapping into one of the world’s fastest-growing aesthetic medicine markets.
The product’s success is largely attributed to its proven quality. In China, only six botulinum toxin products — including Letibot, AbbVie’s Botox, Lanzhou Institute’s Hengli, and Ipsen’s Dysport — have secured NMPA approval, underscoring the barriers to entry and regulatory rigor.
Among over ten Korean botulinum toxin manufacturers, Hugel is the only company with an NMPA-approved product. The company received approval for Letibot 100U in 2020, followed by Letibot 50U in 2021.
According to market research firm Frost & Sullivan, China’s botulinum toxin market is projected to grow from KRW 2.5 trillion in 2024 to KRW 7.7 trillion by 2030, nearly tripling in size over five years. Experts predict that as the market matures and consumer demand becomes more mainstream, products with verified safety and efficacy will gain even greater traction.
Alphabiz Reporter Kim Jisun(stockmk2020@alphabiz.co.kr)