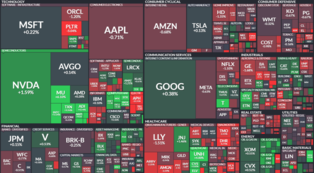
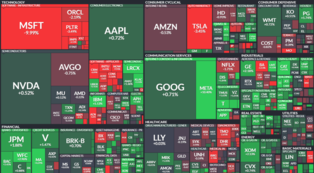
[Alpha Biz= Reporter Kim Sangjin] Hana Securities analyzed on the 10th that Genomictree’s potential revenue from its bladder cancer early detection product, EarlyTEK-B, is expected to be conservatively around 40 billion to 230 billion KRW starting from next year, as the product moves toward full-scale entry into the U.S. market.
In a report released that day, Hana Securities explained, “After EarlyTEK-B was designated as an innovative medical device by the U.S. FDA in April 2023, it entered the U.S. market in June of the same year through Laboratory Developed Test (LDT) services without FDA approval.” The firm added, “In April this year, the product received a CPT-PLA medical code from the American Medical Association (AMA), and on the 3rd of this month, the Centers for Medicare & Medicaid Services (CMS) set the test price at $192, completing preparations for commercialization.”
EarlyTEK-B is expected to be officially included in the AMA's CPT codebook starting in January 2025, and the submission of data for the MolDx program, which is required for Medicare coverage registration, is also planned. This is expected to accelerate the adoption of the test by U.S. insurers and secure a competitive advantage in the bladder cancer early detection market.
Hana Securities further analyzed, “Approximately 82,000 people are diagnosed with bladder cancer each year in the U.S., and it is estimated that about 10% of these patients are eligible for early detection tests. Based on this, over 820,000 bladder cancer tests are expected to be conducted annually, with EarlyTEK-B’s potential revenue estimated to be between 40 billion and 230 billion KRW (based on exchange rates).”
The firm also noted, “Similar to how Pacific Edge’s stock price surged after launching LDT services in New Zealand, Genomictree is expected to experience similar growth in the U.S. market. Especially since Genomictree is currently the only company offering an LDT service for early bladder cancer detection in the U.S., it is likely to establish a dominant position in the market.”
In addition, the company’s EarlyTEK-C, an early detection product for colorectal cancer, is also in the final stage of commercialization. In November, a large-scale confirmatory clinical trial involving 2,358 high-risk patients was completed in South Korea, and the results report is expected to be submitted soon.
Alphabiz Reporter Kim SangJin(letyou@alphabiz.co.kr)