 |
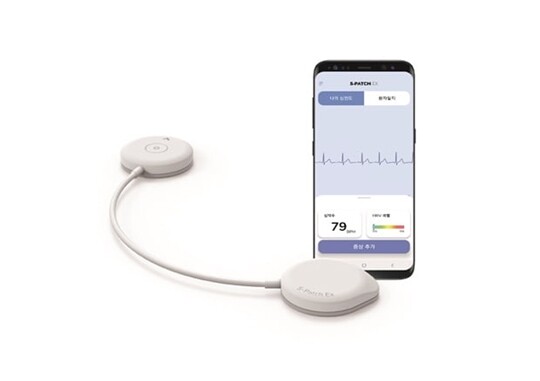
[Apha Biz=(Chicago) Reporter Paul Lee] Samjin Pharmaceutical announced on the 6th that its wearable ECG "Spatch-EX" has been approved by the US Food and Drug Administration (FDA).
Dispatch-EX is developed by Wellisis, a digital healthcare startup spun off from Samsung SDS, and Samjin Pharmaceutical is in charge of domestic sales. It is a light and small wearable ECG device with a thickness of 6㎜ and a weight of 9g, consisting of Samsung SDS's software, Samsung Electronics' semiconductor chip bioprocessor, and algorithms developed by Samsung Hospital.
Samjin Pharmaceutical explained that Dispatch-EX can solve the inconvenience of ECG tests experienced by patients and medical staff.
Dispatch-EX can record a user's symptoms with a mobile application. The recorded symptoms are automatically displayed in the software, making it easier to manage patients, the company explained. After the examination, the data is sent to the cloud server and can be diagnosed regardless of time. The number of visits to hospitals by patients can also be adjusted according to their medical conditions.
Alphabiz Reporter Paul Lee(hoondork1977@alphabiz.co.kr)