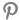|
| Children's fever reducer which was recalled due to crystallization. (Photo: Korea Food and Drug Administration) |
[Alpha Biz= Reporter Kim Sangjin] According to the Korea Food and Drug Administration, some syrup-type children's fever relievers sold in pharmacies have been found to contain crystallization, prompting a recall order.
On the 24th, according to the administration, certain products of "NaeRin Syrup (active ingredient: acetaminophen)" produced by TELCON RF PHARMACEUTICAL and sold by Kwangdong Pharmaceutical have exhibited crystallization in the liquid, leading to ongoing recall procedures by the distributors.
The products subject to recall have the manufacturing number 23001 and an expiration date of February 26, 2025.
An official from the administration stated, "We are currently investigating the cause of the crystallization."
Alphabiz Reporter Kim SangJin(letyou@alphabiz.co.kr)