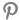|
[Alpha Biz=(Chicago) Reporter Paul Lee] The market closed sharply on the 19th after news that Shin Poong Pharm had failed to phase III clinical trials for a new coronavirus infection (COVID-19) drug under development.
According to the Korea Exchange, Shin Poong Pharm closed at 11,030 won, down 18.96% from the previous trading day.
This can be attributed to news that the efficacy of the COVID-19 treatment Pyramax tablet, which is being developed by Shin Poong Pharm, has not been proven in Phase III global clinical trials.
Shin Poong Pharm said in its clinical phase 3 top line results released after the closing of the market the previous day that the primary validity evaluation variables for suppressing the severity rate in adult patients with symptoms were not met.
Alphabiz Reporter Paul Lee(hoondork1977@alphabiz.co.kr)