[Alpha Biz=(Chicago) Reporter Kim Jisun] HLB, which received official approval from the U.S. Food and Drug Administration (FDA) for the main review of the New Drug Application (NDA) for Rivoceranib, a liver cancer treatment, took out a bonus issue card to respond to short selling.
HLB announced on the 19th that it had decided on a bonus issue, allocating 0.05 shares per share of common stock and other stocks. As for uncertified resources, 1500% of capital surplus was used as of the first quarter. New shares will be listed on August 21st.
The ostensible reason for this bonus issue is a shareholder-friendly policy following the approval of the main review for Rivoceranib's new drug approval.
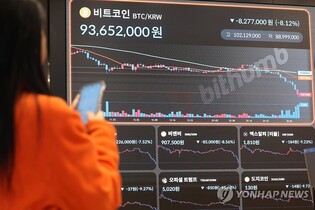
However, in reality, it is interpreted as a strong will to respond to the forces of short selling. The share price of HLB rose to 38,000 won in June and is currently trading at 30,000 won as of the 18th.
As of the 13th, the short sale balance of HLB was about 200 billion won, ranking 4th among KOSDAQ stocks. In the past, Celltrion also conducted a free issue to respond to short selling forces.
Alphabiz Reporter Kim Jisun(stockmk2020@alphabiz.co.kr)