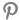|
Photo = Yonhap news |
[Alpha Biz= Paul Lee] LG Chem announced on the 27th that it has voluntarily discontinued the global Phase 3 clinical trial for 'LC350189', a treatment for gout patients with hyperuricemia.
Previously, LG Chem had received approval for the LC350189 clinical trial from regulatory agencies in 21 countries, including South Korea, the United States, and Europe. The treatment had demonstrated safety and superiority over a placebo in clinical trials conducted last year.
LG Chem explained that the decision to halt the Phase 3 trial was made in line with a strategic resource reallocation decision, considering the commercial value of the treatment.
Alphabiz Reporter Paul Lee(hoondork1977@alphabiz.co.kr)