|
Photo courtesy of Yonhap News |
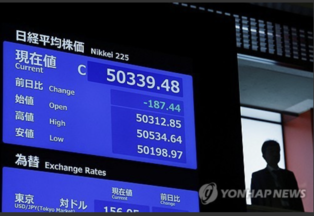
[Alpha Biz= Kim Jisun] SEOUL – Yuhan Corporation’s lung cancer therapy Leclaza (lazertinib, marketed as Lazcluze overseas) has secured regulatory approval in China, marking another milestone for the drug now authorized in the U.S., Europe, Japan, and the U.K. But despite growing approvals and milestone inflows, royalty revenues remain below expectations.
According to industry sources on July 31, Janssen, which holds the global rights to Leclaza, received Chinese NMPA approval on July 29 for Lazcluze (JXHS2400009) in combination with Rybrevant (amivantamab) for patients with EGFR-mutated non-small cell lung cancer (NSCLC).
The Leclaza-Rybrevant combo was previously approved in the U.S. and Europe last year, and this year in Japan and the U.K.. Yuhan booked ₩20.7 billion in milestone revenue in Q2 from the Japan approval alone.
However, royalty income from commercial sales remains modest. In Q2, Yuhan reported ₩25.5 billion in licensing revenue — up 40x year-over-year — but nearly all came from milestones. Royalties accounted for just ₩3.4 billion (₩2.0 billion net to Yuhan after sharing 40% with Oscotec and Genosco), only 0.6% of total sales (₩556.1 billion).
This fell short of market hopes after Johnson & Johnson reported USD 179 million (~₩250 billion) in Q2 sales from the Lazertinib–Rybrevant combo, triggering a 22% surge in Yuhan’s share price at the time.
Analysts flagged the slower-than-expected royalty ramp-up:
NH Investment & Securities’ Han Seung-yeon cut Yuhan’s 2024 royalty forecast from ₩32.4 billion to ₩20.5 billion, saying Q2 royalties of ₩3.4 billion missed the firm’s ₩5.5 billion estimate.
Kiwoom Securities’ Heo Hye-min noted royalties are “flowing in more slowly than expected,” but expects faster U.S. penetration after FDA approval of the subcutaneous (SC) formulation of Rybrevant and publication of updated survival data.
Alphabiz Reporter Kim Jisun(stockmk2020@alphabiz.co.kr)