 |
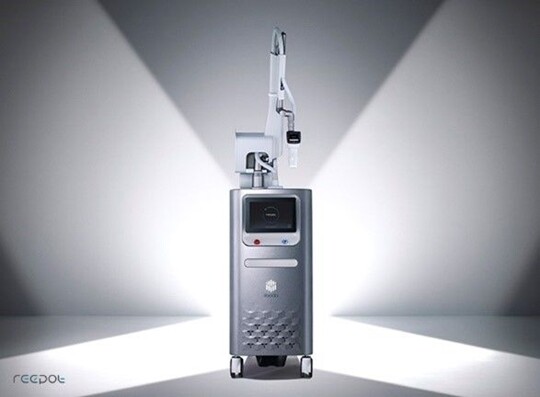
[Alpha Biz=(Chicago) Reporter Kim Jisun] ILOODA, a company specializing in skin beauty medical devices, said on the 30th that it has obtained certification for its flagship product "reepot" from the Saudi Food and Drug Safety Organization (SFDA).
The reepot is a medical device to which the 532㎚ wavelength Q-Switched Nd (YAG) laser source is applied with "VSLS" technology. Major core technologies such as contact cooling CPTL®, cooling control technology, and AutoDerm® image processing technology are applied.
reepot obtained product approval from the Ministry of Food and Drug Safety in April last year after a successful launch in Korea. In September last year, it was approved by the US Food and Drug Administration (FDA). Not only has the safety and quality of the product been proven through domestic and international certification, but it also has confidence in product development.
Alphabiz Reporter Kim Jisun(stockmk2020@alphabiz.co.kr)